Background: Marginal Zone Lymphoma (MZL) is considered indolent but incurable, with a variety of initial treatment strategies including observation. Quality of life (QOL) can be affected by disease burden, side effects of treatment, and psychosocial effects of living with an incurable cancer. However, little is known about long-term QOL in patients with MZL. We investigated QOL at baseline (BL), and up to nine years after a diagnosis of MZL.
Methods: Newly diagnosed MZL patients aged 18 years and older were prospectively enrolled within 9 months of diagnosis in the Iowa/Mayo Clinic Molecular Epidemiology Resource from 9/2002 - 6/2015. Pathology was reviewed by a hematopathologist. All MZL participants with both BL and at least one follow-up (FU) QOL questionnaire were eligible for this analysis. QOL was measured at BL and years 1-, 2-, 3-, 6- and 9-year FU with the Functional Assessment of Cancer Therapy-General (FACT-G) scale (range 0-108), which measures 4 QOL domains (range): physical (0-28), social/family (0-28), emotional (0-24), and functional (0-28) well-being (WB). Clinical data were abstracted according to a standard protocol and patients were systematically followed for events (progression, re-treatment, and death). Patients were grouped into initial management with active surveillance (observation), local/antibiotic therapy (including radiation, surgery, and anti- H. pylori therapy), or systemic therapy (including chemotherapy, targeted agents, or antibody therapy). Change in QOL scores over time was analyzed using mixed models for repeated measurements, and comparisons were made according to initial treatment group.
Results: Of 324 participants in the analysis, 59% were female, 98% were White and the median age was 63 years. For initial management, 36% (N=118) were observed, 30% (N=96) received local/antibiotic therapy and 34% (N=110) received systemic therapy. Across the initial treatment groups, there were no statistically significant differences by age, gender, or performance status, while there were differences for Ann Arbor Stage IV (observation 50%, local/antibiotic 27%, systemic 67%, p<0.001) and MALT-IPI score 2+ (observation 23%, local/antibiotic 18%, systemic 28%, p<0.001).
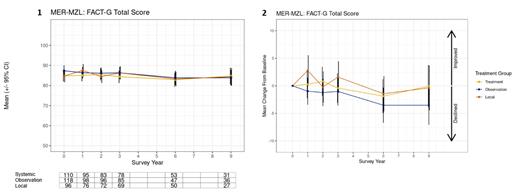
At BL, mean FACT-G total score was 85.7 (SD 13.1), and physical, social/family, emotional, and functional WB scores were 20.3 (SD 4.2), 24.4 (SD 3.9), 18.9 (SD 3.5), 22.1 (SD 5.3); these scores were similar across initial treatment groups (all p>0.05). FACT-G total score was largely stable over time by treatment group (Panel 1). However, it declined at years 6 (mean -4.0, p<0.05) and 9 (mean -3.7, p<0.05) in the observation group (Panel 2), driven by a decline in functional WB, although this change was not significantly different from the other treatment groups. Further, in comparison with other treatment groups, local therapy was associated with a short-term 1-year improvement of FACT-G total score (mean +2.8, p=0.04), driven by improvements in both functional and emotional WB.
For the subscales, we identified two major trends irrespective of treatment group: an overall improvement from BL of emotional WB at years 1 (mean +0.6, p<0.05), 2 (mean +0.7, p<0.05), 3 (+1.3, p<0.05), 6 (+1, p<0.05), and 9 (+1.3, p<0.05) and an overall decline of social/family WB at years 1 (mean -1.1, p<0.05), 2 (mean -1.7, p<0.05), 3 (-2.1, p<0.05), 6 (-2.7, p<0.05), and 9 (-2.2, p<0.05). There was no notable change from BL for physical or functional WB.
Conclusions: This analysis is the first to provide prospective real-world QOL data on a longitudinal cohort of MZL patients with long follow-up. At BL, initial treatment was associated with stage and MALT-IPI score, but not QOL. Except for the transient 1-year QOL improvement in patients under local/antibiotic therapy, initial treatment groups had no impact on QOL over time. Irrespective of treatment group, emotional WB showed improvement over time, suggesting psychosocial adaptation by the patient, as it was found in aggressive lymphomas (Thompson et al., 2018). Further, emotional WB over time did not vary across treatment groups, suggesting that being initially observed did not lead to more emotional stress than being actively treated. Finally, decline in social/family WB (support from friends, family, family acceptance of illness, family communication, close to partner) suggests a need to consider interventions to patients and families to address these needs.
Disclosures
Bommier:Philippe Foundation: Other: Mobility funding; LYSA/ELI: Research Funding; ITMO/AvieSan: Research Funding; INSERM: Current Employment; Institut Servier: Research Funding. Tsang:Poseida Therapeutics: Current holder of stock options in a privately-held company. Casulo:SecuraBio: Research Funding; Gilead Sciences: Research Funding; Follicular Lymphoma Foundation: Other: Leadership role; GenMab: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Verastem: Research Funding; Abbvie: Consultancy; Genentech: Consultancy, Research Funding; Lymphoma Research Foundation: Other: Leadership Role. Maurer:BMS: Consultancy, Research Funding; Roche/Genentech: Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Habermann:sorrento: Research Funding; Genentech: Research Funding; BMS: Research Funding. Cerhan:Genmab: Research Funding; NanoString: Research Funding; Protagonist: Other: Safety Monitoring Committee; BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal